Radiopharmaceuticals: a new class of drugs for cancer patients, and a new road for Canada to lead global life sciences
November 26 2020
The role of radiopharmaceuticals in health care may still be quite a mystery for many Canadians, but this area has a tremendous therapeutic and commercial potential in our country. Canada is already a global leader in radioisotope-based health care technologies, meaning it has the strengths to also become a world leader in bringing related novel therapeutics to patients.
During the next few weeks, adMare BioInnovations and the Centre for Probe Development and Commercialization (CPDC) will present you with a series of blogs about this promising field. You’ll discover the potential benefits of radiopharmaceuticals, the current Canadian landscape and, last but not least, the CPDC & adMare Radiopharmaceutical Initiative (CARI), and how it can be the path towards translating radiopharmaceutical discoveries to the clinic.
What are radiopharmaceuticals?
Radiopharmaceuticals are drugs that contain radioactive material called radioisotopes1. They are used to diagnose or treat cancer. A subclass of these, called radioconjugates, consist of four components: radioisotope, ligand and linker with a chelator attached to it to bind the radioisotope2.
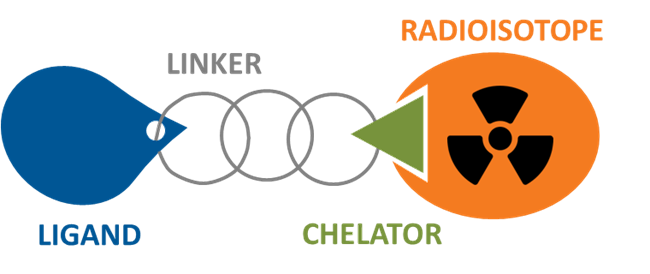
Radioisotopes are atoms that emit radiation, which can be used to provide an image (radiodiagnostic agent) or are used as therapeutics (radiotherapeutic agent) to kill or stop the growth of the diseased cells/tissue3. Radioisotopes used in radiopharmaceuticals are selected based on the type of radiation emitted which can be grouped into three types of radiation including alpha, beta, or gamma radiation. Alpha and beta radiation are primarily used in radiotherapeutic agents because the radiation is able to damage and kill diseased cells but don’t travel very far4. On the other hand, gamma radiation travels farther and is used in radiodiagnostic agents because gamma radiation can be detected using specialized cameras4. Ligands are molecules that interact with cellular proteins, such as receptors2. For example, using a ligand that is attracted to receptors overexpressed in the tumors but not in healthy cells is a strategy used in radiopharmaceuticals2. Lastly, a chemical linker that also comprises a chelator compound attaches the ligand to a radioisotope. A chelator is a compound that binds to the radioisotope and helps with stability.
In general, radiopharmaceuticals are like microscopic heat seeking missiles that target areas of the ligand of interest2. They basically are targeted radiotherapies. An emerging new field, called theranostics, utilizes the pairing of a radiodiagnostic and radiotherapeutic agents, essentially using the same ligand, linker and chelator while changing the radioisotope component of the drug, for diagnosis and treatment of the disease, respectively.
What are the advantages of radiopharmaceuticals?
The advantage of radiopharmaceuticals over traditional pharmaceuticals is that the radioactivity from the radioisotope allows for non-invasive monitoring or targeted therapeutic irradiation with very little effect on normal biological processes, resulting in excellent safety records. Radiodiagnostics are used to enable physicians to see biochemical activity of disease cells, to diagnose or stage a disease, identify which patients are best suited for a particular treatment and help monitor a patient’s response to treatment3.
Unlike chemotherapy, where radiation is non-selectively applied to the body, new radiopharmaceuticals are being designed to precisely target and kill cancer cells, while sparing normal healthy tissues and clearing rapidly from body3.
In addition to the safety aspect, a second advantage of radiopharmaceuticals is that the target protein (e.g. receptor) is not required to induce a pathophysiological outcome by being activated/inhibited/modulated by the ligand as a pharmaceutical agent alone would necessitate. Rather, with radiopharmaceuticals the efficacy results from the direct action of radiation-induced damage and the consequent death of cancer cells
Success Story: Lutathera®
Lutathera is a radiopharmaceutical marketed by Norvartis is known as a successful case study due to its treatment results and sales around the world in a very short period. It was the first Peptide Receptor Radionuclide Therapy (PRRT) to receive regulatory registration, with approval by the European Commission in September 2017 and by the US Food and Drug Administration (FDA) in January 2018.
It targets tumours that express certain proteins on their surface, called somatostatin receptors, in order to selectively deliver radioactivity to the tumour5. The radioactivity from the radioisotope causes damage and eventually kills cancer cells while leaving normal cells intact5. It is the first and only therapeutic radiopharmaceutical indicated for the treatment for somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs)6, which affect approximately 12,000 people in the United States7 and 2,300 people in Canada each year8,9.
“Treatment options have been limited for patients with neuroendocrine tumors and toxicities of treatment can often outweigh the benefit. Studies have shown Lutathera is an effective option to treat tumor progression and also provide patients with a better quality of life,” – Dr. Jonathan Strosberg, head of Neuroendocrine Tumor Program at H. Lee Moffitt Cancer Center & Research Institute, located in Tampa, Florida, 7
During 2018, Lutathera sales were reported to have amounted to $167M USD, dosing around 2,300 patients10. Over 100 centers were actively prescribing this treatment and reimbursement was achieved in several European countries 10. One year later, Lutathera continued to grow with over 170 centers, actively treating patients, and sales were reported to have reached $441M USD11. Within Canada, the treatment has already been approved for reimbursement in Quebec and Ontario, allowing Canadian patients to have access to this radiopharmaceutical therapy5,12.
In the next part of the blog series we will focus on the Canadian radiopharmaceutical landscape, including a snapshot of the tremendous strengths we’ve already built in this country that can be leveraged as a therapeutic driver for patients, and an economic driver for the country.
About CARI
The CPDC & adMare Radiopharmaceutical Initiative (CARI) was launched to bring respective resources together in order to advance the radiopharmaceuticals area in Canada. The objective of CARI is to seek out and uncover radiopharmaceutical opportunities arising from academic discoveries and early enterprises, establish rigorous drug development programs around selected opportunities, and drive the generation of proof-of-principle and other data supportive of the commercialization of new radiopharmaceutical assets.
Click here to learn more and apply for CARI.
References
- https://www.iaea.org/sites/default/files/55405811013.pdf
- https://www.pointbiopharma.com/science
- https://www.imagingprobes.ca/about/what-are-radiopharmaceuticals/
- https://www.adacap.com/nuclear-medicine/
- https://www.newswire.ca/news-releases/radiopharmaceutical-lutathera-r-now-reimbursed-in-quebec-for-cancer-patients-with-advanced-gastroenteropancreatic-neuroendocrine-tumours-gep-nets–843327349.html#
- https://www.cancer.org/latest-news/fda-approves-lutathera-for-certain-digestive-tract-cancers.html
- https://www.eurekalert.org/pub_releases/2018-06/hlmc-ntp061218.php
- https://survivornet.ca/connect/partners/carcinoid-neuroendocrine-tumour-society-canada-cnets-canada/
- http://www.bccancer.bc.ca/health-professionals/clinical-resources/cancer-management-guidelines/gastrointestinal/neuroendocrine-tumors
- https://www.novartis.com/sites/www.novartis.com/files/q4-2018-ir-presentation.pdf
- https://www.novartis.com/sites/www.novartis.com/files/q4-2019-investor-presentation.pdf
- https://www.newswire.ca/news-releases/radiopharmaceutical-cancer-treatment-lutathera-r-now-available-to-ontario-patients-with-midgut-neuroendocrine-tumours-nets–892051767.html




